Physical properties of d-block elements
Physical properties of d-block elements
Key points: The properties of the d metals are largely derived from their electronic structure, with the strength of metallic bonding peaking at Group 6; the lanthanide contraction is responsible for some of the anomalous behaviour of the metals in the 5d series.
The d block of the periodic table contains the metals most important to modern society.
It contains the immensely strong and light titanium, the major components of most
steels (Fe, Cr, Mn, Mo), the highly electrically conducting copper, the malleable gold and
platinum, and the very dense osmium and iridium. To a large extent these properties derive
from the nature of the metallic bonding that binds the atoms together.
Generally speaking, the same band structure is present for all the d-block metals
and arises from the overlap of the (n 1)s orbitals to give an s band and of the nd orbitals
to give a d band. The principal differences between the metals is the number of electrons
available to occupy these bands: Ti (3d24s2) has four bonding electrons, V (3d34s2) five,
Cr (3d54s1) six, and so on. The lower, net bonding region of the valence band is therefore
progressively filled with electrons on going to the right across the block, which results
in stronger bonding, until around Group 7 (at Mn, Tc, Re) when the electrons begin to
populate the upper, net antibonding part of the band. This trend in bonding strength is
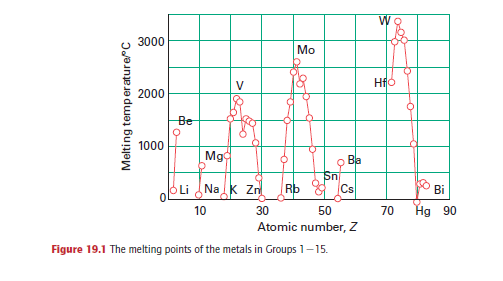
reflected in the increase in melting point from the low-melting alkali metals (effectively
only one bonding electron for each atom, resulting in melting points typically less than
100ºC) up to Cr, and its decline thereafter to the low-melting Group 12 metals (mercury
being a liquid at room temperature, Fig. 19.1 . The strength of metallic
bonding in tungsten is such that its melting point (3410ºC) is exceeded by only one other
element, carbon.
The radii of d-metal ions depend on the effective charge of the nucleus, and ionic radii
generally decrease on moving to the right as the atomic number increases. The radius of
the metal atoms in the solid element is determined by a combination of the strength of the
metallic bonding and the size of the ions. Thus, the separations of the centres of the atoms
in the solid generally follow a similar pattern to the melting points: they decrease to the
middle of the d block, followed by an increase back up to Group 12, with the smallest
separations occurring in and near Groups 7 and 8.
The atomic radii of the elements in the 5d series (Hf, Ta, W,...) are not much bigger
than those of their 4d-series congeners (Zr, Nb, Mo,...). In fact, the atomic radius of Hf
is smaller than that of Zr even though it appears in a later period. To understand this
anomaly, we need to consider the effect of the lanthanoids (the first row of the f block).
The intervention of the lanthanoid elements in Period 6 corresponds to the occupation
of the poorly shielding 4f orbitals. Because the atomic number has increased by 32
between Zr in Period 5 and its congener Hf in Period 6 without a corresponding increase
in shielding, the overall effect is that the atomic radii of the 5d-series elements are much
smaller than expected. This reduction in radius is the lanthanide contraction introduced
in Section 1.9a. The lanthanide contraction also affects the ionization energies of the
5d-series elements, making them higher than expected on the basis of a straightforward
extrapolation. Some of the metals—specifically Au, Pt, Ir, and Os—have such high ionization
energies that they are unreactive under normal conditions.
Atomic mass increases with atomic number, and the combination of this increase with the
changes in the radii of the metal atoms in the metal lattice means that the mass densities of
the elements reach a peak with Ir (density 22.65 g cm 3). Figure 19.2 illustrates this trend.





Comments
Post a Comment